Prostate Cancer Research News
Learn the latest about prostate cancer detection, diagnosis and treatment
Rogel Cancer Center Researchers Discover Gene that Drives Prostate Cancer
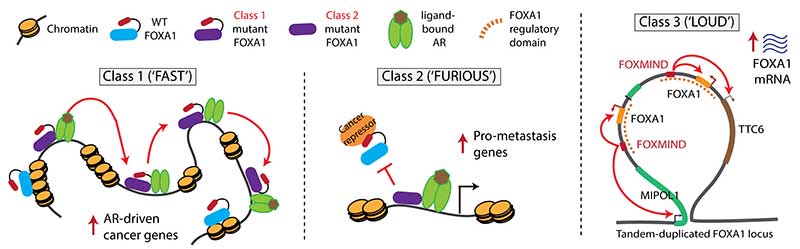
Researchers characterize three ways in which the gene FOXA1 mutates to trigger prostate cancer
The Fast and the Furious movie franchise meets the Fast N’ Loud television series to define an oncogene that drives 35% of prostate cancers.
A new study from researchers at the University of Michigan Rogel Cancer Center finds that the gene FOXA1 overrides normal biology in three different ways to drive prostate cancer. They refer to the three classes as FAST, FURIOUS, and LOUD to reflect their unique features. The findings are published in Nature.
“It’s quite intriguing and complex biology,” says senior study author Arul M. Chinnaiyan, M.D., Ph.D., director of the Michigan Center for Translational Pathology and S.P. Hicks Endowed Professor of Pathology at Michigan Medicine.
“We found that the same gene can be turned into an oncogene in three different ways,” says Abhijit Parolia, a molecular and cellular pathology graduate student and co-first author on this study. “One moves fast in the nucleus, the second binds to chromatin furiously and the third amplifies itself to be loud. These three alteration classes have different clinical implications for patients.”
Class 1 mutations are FAST. They cause the transcription factor to travel more quickly through the DNA, allowing the partnering androgen receptor to activate expression of cancer-promoting genes. Imagine the driver racing forward at high speed. These mutations are seen in early stage prostate cancer and are likely what triggers the disease.
Class 2 mutations are FURIOUS. The mutation causes a portion of the FOXA1 molecule to be cut off. This truncated molecule binds very strongly to the DNA, preventing normal FOXA1 from binding. These mutations are found in lethal hormone-therapy resistant prostate cancer and promote the cancer’s spread to distant sites. Think of the mutant as furiously binding DNA and dominantly enabling the cancer’s aggressive features.
Class 3 mutations are LOUD. They involve complex rearrangements of the FOXA1 genomic position, creating duplications in which FOXA1 or other oncogenes are overexpressed. In other words, the amplified oncogenes work at top volume to be biologically heard. This can occur in both early stage and metastatic cancer.
Long-term hormone therapy increases mortality risk for men with low PSA levels after prostate surgery
Analysis finds PSA levels predict which men with recurrent prostate cancer will be harmed by adding long-term hormone therapy to radiation
A secondary analysis of a recent clinical trial that changed the standard of care for men with recurring prostate cancer finds long-term hormone therapy does more harm than good for many men and calls for rethinking treatment guidelines based on a patient’s post-operative prostate-specific antigen (PSA) level. Findings were presented at the 61st Annual Meeting of the American Society for Radiation Oncology (ASTRO) in Chicago.
The study reanalyzed data from NRG Oncology/RTOG 9601, a randomized, phase III clinical trial initially reported in 2017 that found adding two years of anti-androgen therapy to post-surgical radiation treatment for men with recurring prostate cancer increased their long-term overall survival rate. That study led to the recommendation that men with recurrent prostate cancer be treated with both radiation and long-term hormone therapy after surgery.
However, a secondary analysis of this data, splitting patients into those with high and low PSA levels, has found that men with low PSAs after prostate surgery gained no overall survival benefit from long-term hormone therapy and greatly increased their risk of dying from other causes
U-M, Karmanos receive $9.2M grant for prostate cancer research
Collaborative projects will address key questions about how prostate cancer develops and how best to treat it
Michigan’s two elite cancer programs are joining forces to find new solutions for prostate cancer. The University of Michigan Rogel Cancer Center and the Barbara Ann Karmanos Cancer Institute at Wayne State University have received a prestigious $9.2 million grant from the National Cancer Institute.
The grant is through the NCI’s SPORE, or Specialized Program of Research Excellence, which funds collaborative, interdisciplinary translational cancer research. The Michigan Prostate SPORE will focus on critical questions regarding how prostate cancer develops, with projects designed to address major barriers and challenges in diagnosis, treatment and metastasis.
