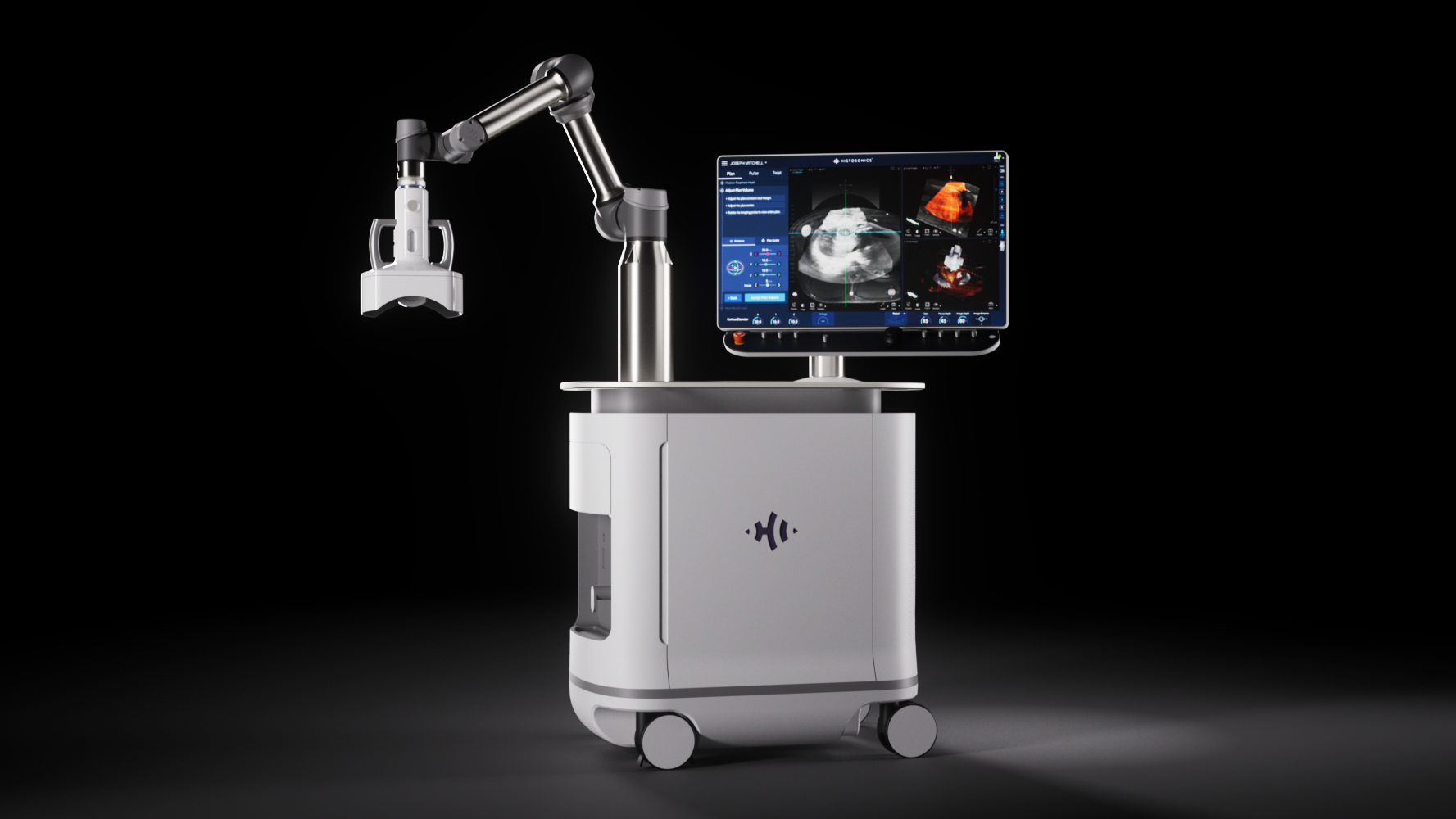
U-M Health to purchase Edison platform for histotripsy, following FDA approval
Media contact: Nicole Fawcett, 734-764-2220 | Patients may contact Cancer AnswerLine™ 800-865-1125
Technology developed at U-M uses sound waves to destroy tissue, providing a new type of cancer therapy
ANN ARBOR, Michigan — University of Michigan Health will purchase the Edison platform used to deliver histotripsy, a technology pioneered at the university that uses sound waves to destroy tissue.
HistoSonics, a company co-founded by U-M faculty, received approval this week from the U.S. Food and Drug Administration to use histotripsy via the Edison platform in liver treatment.
“We are opening a whole new door for cancer therapy, which will hopefully help prolong overall survival in patients,” said Mishal Mendiratta-Lala, M.D., clinical professor of radiology at Michigan Medicine and U-M’s principal investigator of the international clinical trial testing histotripsy.
“We have an amazing team of clinicians, scientists, engineers, and all the students, coordinators and technologists who are involved. The multidisciplinary collaboration we have formed is really what sets our institution apart from anywhere else in the world right now, which is why we have treated the most patients in the world,” she said.
The purchase will be funded through University of Michigan Health, including several U-M departments and centers, as well a generous donation and partnership from Richard and Susan Rogel to help implement the technology.
The Rogels committed $150 million in 2018 to U-M’s cancer center, which is now named the Rogel Cancer Center. Supporting histotripsy was an easy decision, Richard Rogel said.
“I think that our teams have done something incredibly important in creating, developing and testing this technology here at U-M. We are proud to be part of it,” he said.
Pioneered at the University of Michigan, histotripsy offers a promising alternative to cancer treatments such as surgery, radiation and chemotherapy, which often have significant side effects. Histotripsy is a one-time non-invasive treatment that requires general anesthesia only. Patients go home the same day.
Histotripsy uses targeted ultrasound waves to form microbubbles within the tumor. The forces created as those bubbles form and collapse cause the mass to break apart, killing tumor cells and leaving the debris to be cleaned up by the immune system.
Histotripsy relies on focusing acoustic waves of high energy ultrasound to concentrate the energy enough to form bubbles, and the Edison machine can make sure that region is confined to the tumor, so healthy tissue is not affected.
Once the Edison machine is in place, patient care can start almost immediately. Planning has already begun among providers. Histotripsy will be available for liver tumors that can be reached via the ultrasound. For larger tumors or metastatic disease, patients may need chemotherapy or immunotherapy in addition to histotripsy. Treatment plans and management will be individualized for each patient.
A clinical trial is ongoing at the Rogel Cancer Center using histotripsy for primary or metastatic liver cancer. In addition, research continues to explore the technology in renal cancer and other tumors. No other histotripsy clinical trials are currently available at Rogel.
Patients seeking more information about histotripsy, liver cancer treatment or other cancer treatment options may call the Cancer AnswerLine at 800-865-1125.
Disclosure: U-M retains a financial interest in HistoSonics, as do a number of researchers who were involved in this project and who helped develop the technology licensed to HistoSonics. Each stands to benefit financially from the success of the platform. The company was formed with support from Innovation Partnerships, U-M’s central hub for research commercialization.