Researchers discover PanIN lesions may not indicate likelihood of pancreatic cancer
Media contact: Colleen Stone, 734-764-2220 | Patients may contact Cancer AnswerLine™, 800-865-1125
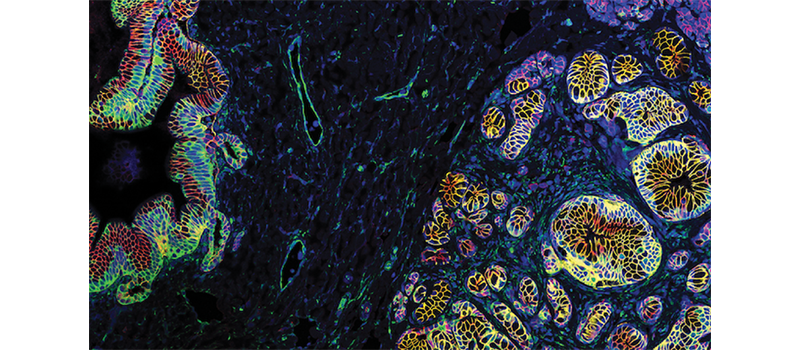
A research partnership between Michigan Medicine and Gift of Life Michigan has revealed a new pathway to understanding the progression of pancreatic cancer.
In a study involving 30 pancreata from donors with no known gastrointestinal disease, researchers found precancerous lesions called pancreatic intraepithelial neoplasia in the majority of the organs. They also discovered that the lesions bore a similar gene expression signature to that of pancreatic cancer. These findings upend conventional wisdom that all PanIN are necessarily precursors to pancreatic cancer; given that the incidence of pancreatic cancer is relatively low, it’s not likely that all (or any) of the lesions are indicators of future cancer.
The findings were published in the journal Cancer Discovery.
According to Timothy Frankel, M.D., an associate professor of Surgery at Michigan Medicine and one of the lead investigators on the paper, the findings will change the way researchers and clinicians think about PanIN lesions — less as indicators of certain future cancer and more as wildcards influenced by the microenvironment surrounding them.
“They’re likely not harbingers of cancer as we thought in the past … Although we see that they are very similar transcriptionally to cancer which suggests that they actually are likely related. What is it that separates the ones that progress to cancer from those that don’t? This work has now opened the door for a decade’s worth of research,” Frankel said.
A research challenge, a serendipitous partnership
Pancreatic cancer research has been hindered by the inability to study the healthy pancreas. There is no indication to biopsy a healthy pancreas, and doing so is risky; the organ is delicate and if ruptured will leak digestive enzymes, causing it to “autodigest,” essentially destroying itself.
As such, establishing a baseline for what a healthy pancreas looks like in the past has involved autopsy studies of normal pancreata or studies of tissue adjacent to tumors. Autopsy studies are limited by the pancreas’ tendency to degrade quickly once cut off from oxygen and blood supply. “Adjacent-normal” tissue is a product of an inflammatory environment, and it turns out is not exactly normal, as it is heavily influenced by surrounding pathology. As such, it often presents with extensive inflammation and other alterations.
Frankel wondered whether colleagues in transplant surgery had insight into acquiring healthy organs for study and Chris Sonnenday, M.D., a transplant surgeon at Michigan Medicine, connected him with the Gift of Life Michigan team at exactly the right time.
The Gift of Life Michigan Donor Care Center, located six miles from Michigan Medicine’s University Hospital, had been in search of pancreatic research partners since the Centers for Medicare & Medicaid Services had emphasized the importance of such research for organ procurement organizations. Nick Olden, research coordinator for Gift of Life, said that organs for which there were no patient recipients were a priority for research matches.
“We want to give every organ and every tissue the chance to be placed with a study and pancreas is a really good example where we just didn't have any projects to support that program,” Olden said.
Marina Pasca Di Magliano, Ph.D., Maude T. Lane Professor of Surgical Immunology at Michigan Medicine and a co-lead investigator on the paper, knew the project would be a big logistical lift. “We had to set up a whole system to get and process the organs because it’s a lot of tissue and it’s very time sensitive. We met with people in the lab and said ‘Anyone who wants in is in this project, but what that means is you have to drop everything you’re doing. If we get a phone call, you have to drop everything.’”
A lot of people signed up, and a lot of people dropped everything when the calls came.
The process the team developed to recover pancreata ensured the organs would be good quality and have blood flow until the last possible moment, nearly eliminating any “warm ischemic” time that contributes to organ degradation. (Earlier attempts to recover organs from donors who’d suffered cardiac death resulted in degraded organs, so the team focused on brain-dead donors to recover organs in a more controlled manner.)
Within just a few months of first making contact, the team had recovered, transported and studied its first pancreas, a process that takes roughly 90 minutes from recovery to receiving.
Paradigm-changing findings
It didn’t take long for the project to reveal striking findings. PanIN lesions were found in the first pancreas sampled. Ultimately, 18 of 30 donor pancreata harbored PanIN.
“The number of lesions, particularly in younger individuals, was striking. These donors are much younger than in previous autopsy studies, which was historically the only way we've even been able to know what the natural incidence of PanINs are,” Frankel said.
The age of the donors spanned from people in their third decade of life to those in their eighth, and the lesions were seen in many of the young donor pancreata. This finding countered conventional wisdom that the lesions accumulated over time.
PanINs were not only common, they also looked like tumors.
Eileen Carpenter, M.D., Ph.D., a gastroenterologist at Michigan Medicine and a first author on the paper, worked with a team of bioinformatics specialists to investigate the lesions and their surrounding microenvironment.
“When we dove into the nitty gritty of the bioinformatic analysis, the RNA transcriptome of these neoplastic lesions and the genes that they express looked very similar to tumors. Even though they’re not cancer, they look more like cancer than normal cells,” Carpenter said.

Pasca Di Magliano said that the tumors and lesions had such a similar makeup that it made sense to look beyond PanIN lesions themselves to understand whether something might be urging the lesions on toward cancer progression or holding them at benign status.
“What we think now is that a microenvironment surrounding the lesions might play a role in restraining them and not letting them progress,” Pasca Di Magliano said.
The team investigated the microenvironment around PanIN and found it had distinct characteristics differentiating it from tissue from PanIN-free areas of the pancreas, showing a rich area of fibroblasts, myeloid and T cells.
Along the way, the team made other discoveries. Among them, the presence of Acinar-to-ductal metaplasia (ADM) in the donor pancreata. ADM is seen when pancreatic acinar cells differentiate into ductal-like cells with ductal cell traits. The process is thought to happen under stress, and was not previously recognized to occur in humans.
Another surprising finding was how different the true normal pancreatic microenvironment was when compared to what was previously used as the normal microenvironment control — “adjacent-normal” tissue from the area around tumors.
“It turns out that the tumor has effects beyond it. If that is what you're setting your baseline to and that baseline isn’t the right one, you can imagine that all the conclusions might be changed now that we have a true normal,” Carpenter said.
In search of the ‘holy grail’ — and how a diverse donor panel helps
Now that it’s clear PanIN are not only precursors to pancreatic cancer, but prevalent in young, healthy individuals, the team is now solving for “why”: Why, if so many people have lesions, do so few get pancreatic cancer? What is it that restrains or urges on the process?
A cumulation of mutations may be one key, according to Frankel. The team is investigating the profiles of the mutations next to see whether the lesions have few mutations to begin with and develop more over time.
One factor lending dimension to the research is the makeup of the donor profile. Gift of Life is a central facility, transporting donors from all over Michigan. As such, the cohort of donors is more representative of the general population than those who might come from the local area. The makeup of the cohort of 30 donors in this study was 66.6% Caucasian, 33.3% African American and included one Asian donor and another of unknown race.
“One of the most immediate lines of investigation is going to be differences in population by race and urban versus rural location to start looking at environmental and genetic factors. Most previously studied research samples in pancreas cancer are from Caucasians,” Frankel said.
Early detection and intervention is the gold standard in cancer, and the sneaky nature of pancreatic cancer progression has made the road rough for patients. For Carpenter, this is one step toward smoothing it.
“This is really a seminal paper, and there’s going to be a lot more to come. Ultimately, it will be really neat if this can be leveraged for early detection and early intervention,” Carpenter said.
Paper cited: “Analysis of donor pancreata defines the transcriptomic signature and microenvironment of early neoplastic lesions“ Cancer Discovery.
