Glioma Research in Animal Models has Promising Results
Media contact: Ian Demsky, 734-764-2220 | Patients may contact Cancer AnswerLine, 800-865-1125
A combination approach, which included metabolic reprogramming and immunotherapy, led to complete tumor regression in 60% of study mice.
A combination approach to treating a prevalent glioma subtype — including metabolic reprogramming, radiation, chemotherapy and immunotherapy -- led to a complete regression of tumors in 60% of study mice, new research by the University of Michigan Rogel Cancer Center found.
The findings, which appear in the Journal of Clinical Investigation, support the testing of inhibitors of a key cancer metabolite -- D-2-Hydroxyglutarate (D-2-HG) -- in combination with radiation, temozolomide and PD-L1 immune checkpoint inhibitors as a targeted therapy for patients with a common glioma subtype, the researchers say.
“Our study showed that inhibiting D-2-HG, which is elevated in a subtype of gliomas, was able to improve survival when combined with the current standard of care for these patients,” says study first author Padma Kadiyala, a first-year immunology graduate student in the joint lab of lead study authors Maria Castro, Ph.D., and Pedro Lowenstein, M.D., Ph.D. “When an immune checkpoint inhibitor was also included, we saw an even bigger improvement.”
Gliomas are a common type of brain tumor, accounting for about one third of all nervous system cancers. And one-fifth to one-quarter of gliomas have a mutation in the gene encoding isocitrate dehydrogenase 1 (IDH1).
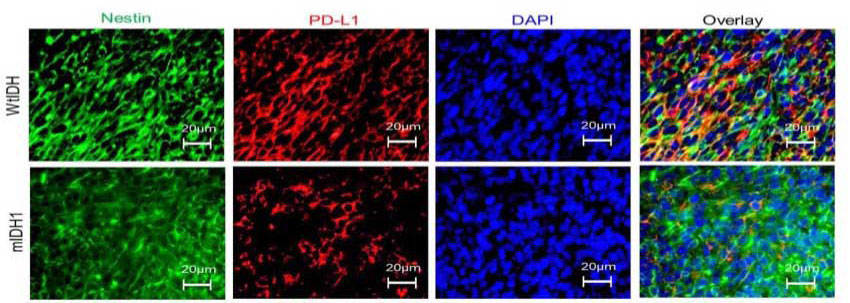
The research specifically looked at a type of astrocytoma -- a subtype of gliomas harboring the IDH1 mutation along with an inactivation of tumor suppressor protein 53 (TP53) gene as well as mutations in another gene called ATRX.
Inhibiting the production of D-2H-G sensitized the tumors to radiation. And it also increased the expression of an immune checkpoint that inhibits T cells’ functions on the surface of the tumor cells. This change was overcome by adding an immune checkpoint inhibitor which improved the immune system’s ability to attack the cancer, leading to long-term survival and anti-glioma immunological memory.
This is critical, since these tumors always recur and are fatal, the researchers note.
“Even when doctors are able to treat these gliomas, recurrence of the cancer remains a major hurdle,” says Castro, a professor of neurosurgery and cell and developmental biology. “These pre-clinical results make us optimistic that we may be able to increase survival and prevent recurrence in patients by using this combination approach to improve the cancer’s sensitivity to radiation therapy, and to reduce T cell exhaustion and generate T cells with a strong immunological memory against the cancer cells.”
Paper Cited: “Inhibition of 2-Hydroxyglutarate Elicits Metabolic-reprogramming and Mutant IDH1 Glioma Immunity in Mice,” Journal of Clinical Investigation. DOI: 10.1172/JCI139542
