Technologies Developed by the Rogel Cancer Center Offer Promise for Early Detection, and Better Monitoring and Management of Cancer
By Ian Demsky
Improving molecular imaging improves early detection of cancer
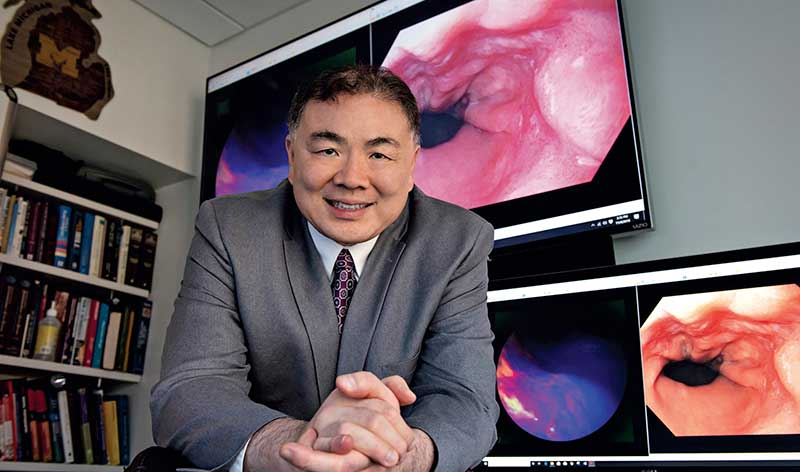
On a screen in his office, Thomas Wang, M.D., Ph.D., watches as an endoscope slides down the pale, smooth tunnel of an esophagus. The tissue is unremarkable, pink and healthy-looking.
"Now watch this," says Wang, a physician-scientist at the University of Michigan, nudging the video forward.
A blue liquid squirts into frame, coating the passage. The lighting shifts from flat, white light to near-infrared light. A patch of cells on one side of the esophagus fluoresces pink and purple -- an indication of pre-cancerous changes invisible to the naked eye.
“Molecular imaging is an emerging technique that has potential to improve the early detection of a variety of cancers -- esophageal, colorectal, stomach, biliary tract, pancreatic, bladder -- that develop in the epithelial tissue of hollow organs,” says Wang, professor of internal medicine, bioengineering and mechanical engineering at U-M.
The liquid contains a peptide developed by Wang’s research team. It selectively binds to receptors on the surface of cells that have undergone transformations in response to chronic stress and genetic changes. In this case, the peptide zeros in on epidermal growth factor receptor (EGFR) proteins, which are overexpressed in high-grade dysplasia, a precursor of esophageal cancer.
Cancer prevention guidelines recommend that patients with high-risk conditions receive traditional endoscopy at regular intervals to look for diseased tissues, along with having random samples from their esophagus biopsied to check for microscopic changes. The instantaneous optical biopsies that Wang’s group is developing would take much of the guesswork out of this process.
“If we can see these damaged cells before they become cancerous, we can resect or ablate them,” Wang says. “It’s much better to prevent cancer than to treat and manage it after it develops.”
U-M is one of three translational research centers across the country that are part of the National Cancer Institute’s Barrett’s Esophagus Translational Research Network (BETRNet) -- a multi-disciplinary, multi-institutional collaboration to centralize and enhance efforts to understand Barrett’s esophagus, another precursor to esophageal cancer.
Along with reporting the first-in-human trials results of the technique for detecting esophageal cancer, Wang’s team has also applied this approach in colorectal cancer, and is in the initial stages of further developing some of the lab’s peptides for broader clinical trials.
They’re also in the process of commercializing a miniaturized microscope they developed -- about 2 millimeters wide -- that can be threaded through an endoscope to analyze tissue samples in real time. Not only does this save the time of sending them out to a pathologist for review, it could often save patients a return visit if something concerning is discovered, he says.
“Engineering an instrument like this is possible because of the amazing resources and cross-disciplinary collaborations that are only available in a place like U-M,” Wang adds.

A wearable biopsy alternative
Wearable devices are increasingly used to monitor many aspects of our heath -- sleep patterns, heart health, menstrual cycles, even alerting us to rising decibels that could damage our hearing. A team of doctors and engineers from U-M believe they could also help to monitor cancer.
The U-M researchers developed a prototype for a wearable device that can continuously collect live cancer cells from a patient’s blood, providing a next-generation alternative to traditional biopsies used to collect and evaluate tumor cells circulating in the blood.
Studying cancer cells captured from blood is known as a liquid biopsy. These are more convenient than a traditional tissue biopsy, requiring only a blood draw, and could provide better information for planning treatments.
Tumors can release more than 1,000 cancer cells into the bloodstream each minute, says Daniel F. Hayes, M.D., Stuart B. Padnos Professor of Breast Cancer Research, who worked on the project. [Note: Dr. Hayes retired from clinical practice in 2023] Many regular blood draws, however, come back with no cancer cells, even in patients with advanced cancer, and a typical sample contains no more than 10 cancer cells.
The new device, about the size of an iPhone, is designed to capture cancer cells continuously over several hours through a catheter inserted into a patient’s vein. In animal models, the cell-grabbing chip in the wearable device trapped three and a half times as many cancer cells per milliliter of blood than the standard approach, according to results published in Nature Communications.
“It’s the difference between a security camera that takes a snapshot of a door every five minutes or one that takes a video. If an intruder enters between the snapshots, you wouldn’t know about it,” says Sunitha Nagrath, Ph.D., associate professor of chemical engineering at U-M, whose lab developed the device.
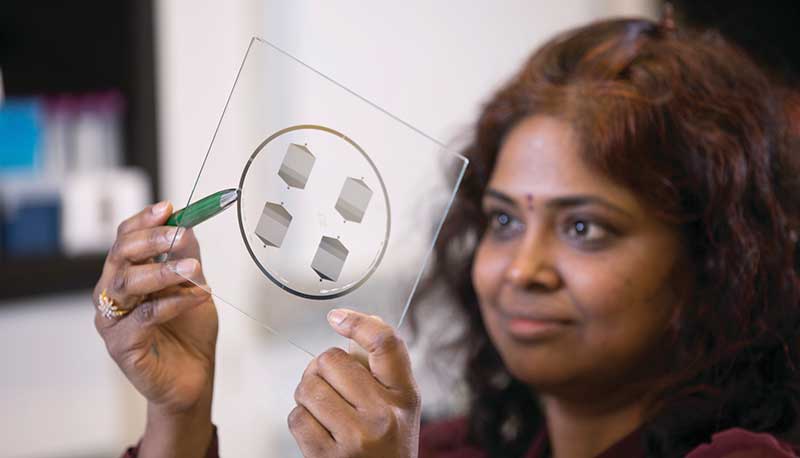
The device is small enough to be worn on the wrist, as opposed to a machine that is typically the size of an oven. A wearable device allows the patient to be mobile during collection. For help with the smaller design, the engineering team turned to Laura Cooling, M.D., professor of clinical pathology at U-M, and associate director of the blood bank, where she manages the full-size systems.
“The most challenging parts were integrating all of the components into a single device and ensuring the blood would not clot, that the cells would not clog up the chip, and that the entire device is completely sterile,” says Tae Hyun Kim, Ph.D., who earned his doctorate in electrical engineering while working in the Nagrath Lab and is now a postdoctoral scholar at the California Institute of Technology.
The team developed protocols for mixing the blood with heparin, a drug that prevents clotting, and sterilization methods that killed bacteria without harming the cell-targeting immune markers, or antibodies, on the chip.

Illustration by Tae Hyun Kim
In the next steps for the device, the team hopes to increase the blood processing rate and estimates the device could begin human trials in three to five years. It would be used to help optimize treatments for human cancers by enabling doctors to see if a patient’s cancer cells might be good candidates for specific, targeted therapeutics.
Setting a trap for cancer cells
In most cancers, the emergence of metastases marks the shift from trying to cure a patient’s disease toward efforts to extend their life.
Jacqueline Jeruss, M.D., Ph.D., a breast cancer surgeon, and Lonnie Shea, Ph.D., a biomedical engineer, have developed a tiny, implantable, porous disc that aims to catch aggressive cells as they first begin to migrate -- long before a cough, pain or other systems reveal a patient’s cancer has overtaken vital organs.
In addition to the potential for early detection of distant recurrence, animal model studies show the implant, which the researchers call a scaffold, may even be able to slow the progress of metastasis by attracting and trapping aggressive cells, thus preventing them from reaching distant organs.
“In my practice, I see patients with aggressive cancer subtypes who are treated with surgery, chemotherapy and radiation, and many of them can be initially rendered disease-free. Unfortunately, some of these patients will eventually develop distant metastasis,” says Jeruss, associate professor of surgery and biomedical engineering. “We know many cancer patients have tumor cells harbored in their bodies, and the immune system may eliminate these cells or the cells remain dormant for many years. We aimed to identify the cancer cells that, over time, graduate to that next stage of aggressiveness and become a metastasis, so we can target them directly.”
The implant is 5 millimeters across -- about the same diameter as a pencil eraser -- and 2 millimeters thick. It is made from a biodegradable polymer called poly(ε-caprolactone), a Food and Drug Administration-approved material used for sutures and wound dressings.

The scaffold is designed to be implanted in the subcutaneous tissue of the abdomen in an outpatient setting using a local anesthetic.
Once installed, the body mounts an immune response to the foreign substance. In healthy mice, the response to the surgery and the device causes some minor inflammation.
“But in mice with cancer, the immune cells in the blood have changed. These immune cells in the blood are drawn to the implant, and as a consequence, this environment then draws in the tumor cells from the vasculature that has grown into the device,” says Shea, William and Valerie Hall Chair of Biomedical Engineering.
The cells captured by the scaffold had significantly more aggressive metastatic properties compared to cells obtained from the primary tumor, and closely resembled cells from lung metastases, the team announced in Cancer Research.
The nature of the implant makes it easy to monitor.
“Using the scaffold, we can ideally monitor the patient’s disease course through ultrasound, core biopsy or by removing the implant -- rather than performing more invasive biopsies of lung, liver or bone,” Jeruss says.
Because they were focused on early detection of distant recurrence and the potential for an analysis of captured cells to recommend or develop targeted therapies, the researchers were intrigued to also find that the implant reduced the cancer burden in solid organs of animal models as well.
Fifteen days after tumor initiation, mice with the implants had 64% fewer cancer cells identified in the liver and 75% fewer cancer cells in the brain, when compared to mice that did not receive the scaffolds, the team reported in 2016.
“It makes sense,” Shea says. “The scaffold is capturing many of those cells and taking them out of circulation.”
A proposal for an initial clinical study of the implant in metastatic cancer patients is under review by the FDA. Shea stresses the device’s additional potential to help tailor immunotherapy to individual patients: “Immunotherapy is taking cancer treatment by storm. There’s a tremendous opportunity to help patients, but this treatment approach is not always successful and it’s not clear why. This device could help provide a window onto an individual patient’s immune function at a metastatic site, to look at what is suppressing the immune system and potentially tailor the nature of the therapy to block that suppression.”
In a blog post, National Institutes of Health Director Francis S. Collins, M.D., Ph.D., called the work a “creative marriage of engineering and medicine,” writing: “[T]his new device… is another promising step in the quest to fight metastatic cancer.”
Learn more about the research and researchers improving cancer detection and monitoring
Print this article ("See Change") or print the 2020 issue
Continue reading Illuminate 2020
Get research news in your inbox!
Our Illuminate e-newsletter showcases the important and unique research underway at the Rogel Cancer Center.
Follow this link and sign-up today!
