Harnessing the Immune System to Target Cancer
By Nicole Fawcett
How cancer immunology moved from a fringe idea to one of the biggest game-changers in cancer treatment

Photo by Edda Pacifico.
When Weiping Zou, M.D., Ph.D., first started looking at cancer immunology, he was one of only a handful in the field who believed in the idea.
“People did not believe the very existence of cancer immunity. The job of the immune system shouldn't be to attack yourself," recalls Zou, Charles B. De Nancrede Professor of Surgery, Immunology, Biology and Pathology at the University of Michigan.
Today, immunotherapy has revolutionized cancer treatment and become one of the most promising avenues in cancer research.
“It’s as big a game-changer as you can imagine,” says Christopher Lao, M.D., MPH, professor of hematology/oncology at Michigan Medicine, who sees patients with melanoma. “There are a number of patients now between five to eight years out with no disease and they’re off treatment. I believe we are curing at least a subset of patients.”
Rogel Cancer Center scientists are uncovering novel approaches in the laboratory to harness the immune system against cancer, and they are working with clinical researchers to translate these ideas into treatment strategies. At the same time, what they learn from patients goes back to the lab to piece together why these treatments work for only a small portion of patients -- and how to expand their success to a larger number.
Fundamental understanding
It was a rocky start. Researchers hypothesized that cancer patients had weakened immune systems and that making the immune system stronger would solve the problem. The focus was on giving patients more T cells, the soldiers of the immune system that do battle against foreign invaders.
These strategies had limited success -- but just enough to keep people believing immunotherapy could work.
“We thought this T cell supplementation strategy wasn’t ideal. Maybe instead we needed to resolve something, find out what’s blocking the immune system,” says Zou, director of the Rogel Cancer Center’s Center of Excellence for Cancer Immunology and Immunotherapy.
Giving patients more T cells would not bolster their immune system if the cancer is putting up a blockade or shield, Zou and others realized. This pushed researchers onto the right track: the discovery that major mechanisms, including CTLA4, PD-1/PD-L1, regulatory T cells and myeloid cells, were putting the brakes on the immune system.
Zou’s lab was first to describe PD-L1 expression and function in the human cancer microenvironment and draining lymph nodes 16 years ago, proposing it as an important suppressive mechanism.
Why the immune system is important
Tapping into the immune system to treat cancer has three main advantages over other therapies:
- It uses the patient’s own body to target and kill the tumor cells, which limits the side effects.
- It can have a good memory, so that once T cells are activated to kill tumor cells, they are able to rev back up if tumor cells pop up again.
- It runs throughout the body, so T cells can track down cancer that metastasizes to different sites.
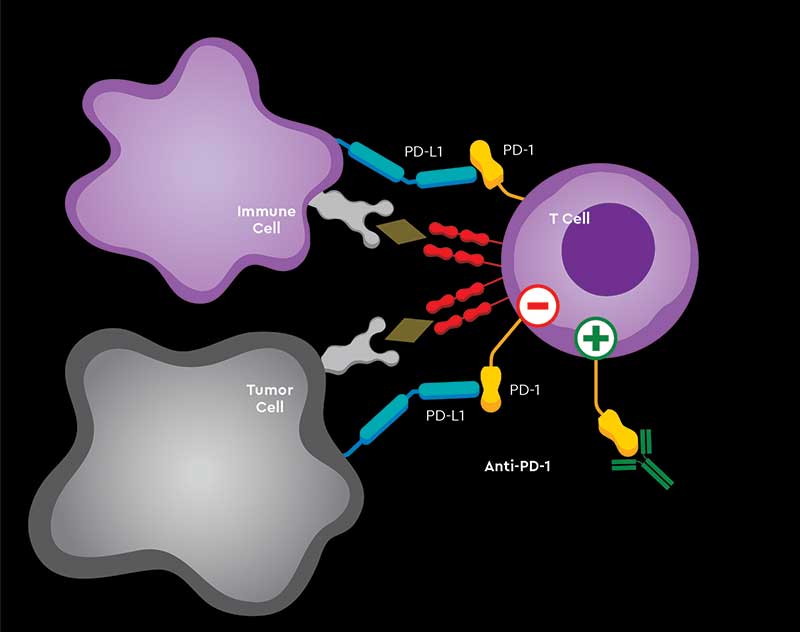
“With targeted therapy, eventually the cancer will develop a resistance mechanism whereby the therapy will no longer work. Whereas with immunotherapy, at least in a minority of patients, once you’ve activated the immune system it may be able to keep the cancer under control for a long time, maybe even years,” says Shirish Gadgeel, MBBS, Mary Lou Kennedy Research Professor in Thoracic Oncology at the Rogel Cancer Center.
The challenge is what Zou calls the yin and yang of the immune system.
“It’s the basic nature of the immune system. It’s been designed to fight against invaders. When you have neoantigens in cancer, they have become invaders and the immune system wants to get rid of them,” Zou says.
So it’s key to mount a strong immune response quickly and efficiently, but then to shut it down. Too much immune activity and the body starts attacking itself, causing immune-related side effects.
Impacting patient care, outcomes
Some of the greatest success with immunotherapy has been seen in stage 4 melanoma and non-small cell lung cancer -- two diseases that have historically had dismal outcomes with few options.
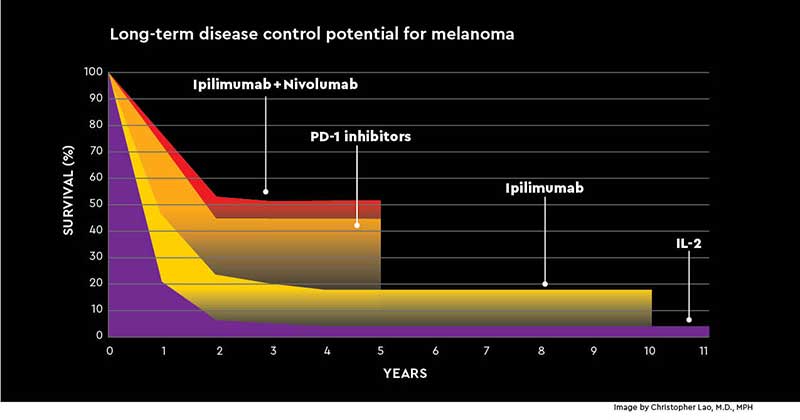
Lao recalls that just a decade ago, he had almost nothing to offer his stage 4 melanoma patients. Today, combination immunotherapy with ipilimumab and nivolumab has changed outcomes dramatically -- from a median survival of six months to more than half of patients living at least five years.
In lung cancer, Gadgeel presented two-year outcomes at the 2019 American Society of Clinical Oncology annual meeting from the KEYNOTE-189 trial, which enrolled more than 600 patients with non-small cell lung cancer. Patients were randomized to pembrolizumab plus platinum-based chemotherapy or chemotherapy alone.
Patients who received chemotherapy and pembrolizumab had a median overall survival of 22 months, compared to 10.7 months for those who received chemotherapy alone. At two years, 45% of patients receiving combination therapy were alive, compared to 29% of patients on chemotherapy alone -- a 16% improvement in overall survival.

Photo by Edda Pacifico.
“It’s a huge difference, especially in lung cancer where until about two years ago the average range of survival was 10–12 months,” Gadgeel says. He has patients who started on the trial in 2014 with stage 4 lung cancer, were treated for two years or less, and now are living with no evidence of disease.
“Admittedly, it’s only a minority of patients. But to have even a minority of patients with no evidence of disease is extremely gratifying. We are very encouraged by these results but by no means satisfied,” Gadgeel says.
Immunotherapy is also standard treatment in kidney and bladder cancer. It’s been approved for Merkel cell carcinoma, squamous cell skin cancer and other cancers.
Cellular therapy
In blood cancers, CAR T-cell therapies are a promising type of immunotherapy. The concept involves taking a patient’s T cells and genetically engineering them to produce receptors on their surface called chimeric antigen receptors, or CARs. The engineered cells are infused into the patient’s bloodstream, where they multiply. The receptors recognize cancer cells and kill them.
“CAR T-cell therapy has the potential to change the face of cancer therapy for years to come,” says Gregory Yanik, M.D., clinical director of U-M’s Pediatric Blood and Marrow Transplantation Program. “This allows us to turn patients’ own cells into a powerful weapon to fight the disease.”
Two CAR T-cell therapies have been approved by the Food and Drug Administration for acute lymphoblastic leukemia and diffuse large B-cell lymphoma. The Rogel Cancer Center was the first center in Michigan to offer all FDA-approved CAR T-cell therapies, and clinical researchers are investigating the therapy in additional cancer types.
Managing side effects
If increased survival is the yin of immunotherapy, the side effects are the yang. Triggering a strong immune response to kill the cancer also leads to immune-related side effects, including rashes, inflamed joints, thyroid or pituitary gland deficiencies, cardiomyopathy, enteritis or colitis, pneumonitis and nephritis.
At the Rogel Cancer Center, oncologists can leverage expertise across Michigan Medicine in fields such as rheumatology, gastroenterology, cardiology and endocrinology -- which have previously had limited engagement with oncology -- to manage these conditions.
“We’re seeing that immunotherapy is a marathon, unlike a sprint with chemotherapy. With chemotherapy, you push as far as possible and then pull back when there are toxicities. With immunotherapy, if you reach toxicities, you need to hold off treatment and recover from them,” says melanoma oncologist Leslie Fecher, M.D., associate professor of hematology/oncology at Michigan Medicine.
“For the most part these toxicities shouldn’t be permanent. With effective management, we should get patients over these,” she says.
Future opportunities
One of the biggest challenges in immunotherapy is that only about 30% of cancer patients overall respond to current checkpoint inhibitors. And among those who do, only a very small portion have sustained long-term responses that have people whispering cure.
Rogel Cancer Center basic and clinical researchers are focused on three key questions about immunotherapy:
- How can we make immunotherapy even more effective to generate more long-term responses?
- How can we make those who are not responding to immunotherapy sensitive to treatment?
- How can we control the immune-related side effects that arise from an active immune system, predicting which patients are at risk and learning how to manage them?
Clinical trials in melanoma, lung cancer and urologic cancers are looking at combining currently available therapies with other approved or new agents to try to expand the pool of responders. Researchers are also testing new immunotherapy agents to add to the arsenal of approved therapies.
“The uniqueness of U-M is that we not only have the capability of doing these early phase trials involving combinations of these agents with targeted therapy, radiation and cytotoxic chemotherapy,” says Lao. “We also have amazing translational scientists working in the laboratory to develop new treatments and biomarkers to assess efficacy, which we can leverage in our trials. With this combination, the hope is we can advance the field and improve outcomes for patients.”
On the laboratory side, Zou’s lab has made fundamental discoveries explaining a suppressive mechanism of regulatory T cells and myeloid cells. In 2019, he published a paper in Nature describing a role for a little-known type of cell death called ferroptosis. When mice were given a checkpoint inhibitor drug in combination with a ferroptosis sensitizer, the impact on tumor growth was dramatically stronger than with either agent alone.
“If ferroptosis is a critical pathway, we may be able to sensitize it to further stimulate immunotherapy or overcome resistance to immunotherapy,” Zou says. “We need to understand this better and work out different mechanisms.”
Using new technology to enhance immune response
One potential way to make immunotherapy more impactful is to change the way it’s delivered. James Moon, Ph.D., John G. Searle Associate Professor of Pharmaceutical Sciences at the College of Pharmacy, has developed a nanodisc to help deliver chemotherapy to cancer cells in a way that tricks the immune system. When combined with a checkpoint inhibitor, the nanodisc eliminated 85% of tumors in a mouse model of colon cancer.
In addition to producing a better immune response, new technologies seek to reduce the immune-related side effects.
“Checkpoint inhibition is amazing but it’s very nonspecific,” says Clifford Cho, M.D., C. Gardner Child Professor of Surgery and chief of hepatopancreatobiliary and advanced gastrointestinal surgery at Michigan Medicine. “Generation 1 is checkpoint inhibition, but generation 2 is going to be targeting more specifically the part of the immune system that’s going after the cancer.”
Image by Clifford Cho, M.D.
Cho is exploring a new technique called histotripsy, which was developed by U-M engineers. It harnesses high-frequency sound waves and focuses them, like a magnifying glass focusing sunlight, into one small point. This causes rapid changes in pressure at the precise point and tears apart cell structures. Because the convergence point is so small and precise, it can be targeted very directly to a tumor without impacting surrounding normal tissue.
What Cho finds most exciting is that when the tumor cells are destroyed, they seem to release hidden proteins and peptides, which suddenly become exposed to the immune system.
“The histotripsy seems to make immunotherapy work even better,” Cho says. “Histotripsy by itself is triggering an immune response, but when we add a checkpoint inhibitor, it’s even more powerful. Our hope is that the immune reaction histotripsy is causing is revving up only an immune reaction to the cancer, not generally stimulating the immune system.”
Cho received a grant from the Forbes Institute for Cancer Discovery at the Rogel Cancer Center to explore this idea further and move it toward translation. The Forbes Institute encourages scientists across the university to undertake highrisk, high-reward initiatives with the potential to drive new advances in cancer research. The intent is to fuel rapid development of innovative technology and new therapies.
Combining immunotherapy and radiation therapy
Laboratory work by Meredith Morgan, Ph.D., associate professor of radiation oncology at Michigan Medicine, is finding a synergy between radiation therapy and immunotherapy. Much like with histotripsy, radiation-induced cell damage releases hidden proteins that suddenly become visible to the immune system. In addition, the DNA damage that radiation causes can lead to DNA leaking from the cell’s nucleus into the cytoplasm, where it is then recognized by the same immune system mechanisms that detect viral DNA following viral infections.
Morgan is using a DNA damage response inhibitor in combination with radiation. The idea: Trick the cell into thinking a virus is attacking. That then triggers the immune system, which would open the door for an immune checkpoint inhibitor to do its job.

“If we inhibit repair of radiation-induced damage, then in theory we should cause more of this damaged DNA to be leaked into the cytoplasm and therefore have greater immune response to tumor cells. The ultimate goal of this is to have a three-way therapy of immunotherapy, radiation and a DNA damage response inhibitor,” Morgan says.
The approach has worked well in the lab, and plans for a clinical trial are underway.
Tracking patients to understand outcomes
Recognizing the need to understand why only some of his patients respond to immunotherapy, Ajjai Alva, MBBS, M.S., began a database he calls the Michigan Retrospective Immunotherapy Experience. It includes data from more than 1,400 cancer patients treated with immunotherapy at the Rogel Cancer Center.
“While we are treating a lot of patients with immunotherapy in everyday practice, we are not really systemically collecting their data to see how are they doing. There are no great biomarkers for immunotherapy,” says Alva, associate professor of hematology/oncology at Michigan Medicine.
The project has led to a number of grants, including a NIH U01 research grant for Alva and Lubomir Hadjiyski, Ph.D., professor of radiology at Michigan Medicine, to look at biomarkers in bladder cancer. Other researchers have also tapped the database to try to answer questions about who does or does not respond to immunotherapy or develop toxicities.
“We are still learning about immunotherapy -- there is much unknown,” Alva says. “But this is going to be a bigger and bigger part of oncology. We’re all immunologists now in one way.”
A New Approach Against Graft-Vs.-Host-Disease
Rogel Cancer Center researchers move laboratory idea through to phase 3 clinical trial
Before checkpoint inhibitors, oncologists were successfully exploiting the immune system in another way: bone marrow transplant.
Here, the biggest issue is balancing the yin and yang of the immune system. That means firing it up enough to kill the cancer cells while limiting graft-vs.-host disease, a potentially deadly side effect rooted in a severely inflammatory environment.

Inflammation led to inspiration for Pavan Reddy, M.D. A 2011 paper suggested alpha-1-antitrypsin, a natural enzyme derived from human blood plasma, had anti-inflammatory effects.
“We asked a very obvious question: If it is really anti-inflammatory and it’s a natural product made by every individual, could we use it to block the graft-vs.-host response?” says Reddy, division chief of hematology/oncology and deputy director of the Rogel Cancer Center.
His lab used alpha-1-antitrypsin in mice that received allogeneic bone marrow transplants and found the drug significantly reduced mortality from graft-vs.-host disease, compared to control mice who did not receive the drug.
In addition, they found alpha-1-antitrypsin reduced the number of inflammatory T-effector cells known to be present in graft-vs.-host disease. It also increased the number of T-regulatory cells, which play a positive role in immune responses.
They published this in 2012 in the Proceedings of the National Academy of Sciences. From there, they advanced the work into a phase 2 clinical trial using alpha-1-antitrypsin in patients with steroid refractory graft-vs.-host disease.
“These patients have a 75–80% mortality and pretty much nothing new being offered to them in terms of treatment options. We knew that if we could make a dent in this population, it would really be significant,” Reddy says.
Results were promising. Of 40 patients evaluated, 26 responded to alpha-1-antitypsin by day 28 -- an overall response rate of 65%. By day 60, 73% of responders continued to see benefit without additional immunosuppression. Overall survival was 45% at six months, compared to 20–30% historically for this population. The study was published in 2018 in Blood.
“We’re very pleased that the toxicity was really not observed at all. That plus the potential for efficacy makes this very exciting,” Reddy says.
A phase 3 national clinical trial will open this year through the Clinical Trials Network, with backing from the National Cancer Institute and the National Heart, Lung and Blood Institute.
The protocol will look at alpha-1-antitrypsin as an upfront therapy to see if adding it to steroids produces better response than steroids alone.
“There’s an endless series of things that are unknown to work on. Hopefully one of them will make a big difference to people,” Reddy says. “You’ve got to keep trying. It takes years and years and years to make a difference.”
Learn more about those researching immunotherapy at Rogel Cancer Center
Continuing reading Illuminate 2020 or print the 2020 issue
Get research news in your inbox!
Our Illuminate e-newsletter showcases the important and unique research underway at the Rogel Cancer Center.
Follow this link and sign-up today!
